Kaken 99Mo-99mTc Process
for Globally Local 99Mo-99mTc production on demand
by combination with TcMM and neighboring reactor or Linac
OUTLINE
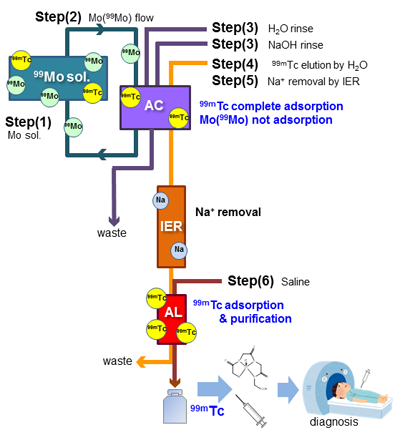
TcMM Proces
- Step(1) Dissolution of irradiated natMoO3 pellets
- Irradiated natMoO3 pellets are dissolved in a molar equivalent NaOH solution, and the resulting Na2Mo(99Mo)O4 solution with the neutral pH can obtain.
- Step(2) Adsorption of 99mTc in AC
- Using the TcMM system, Na2Mo(99Mo)O4 solution (max. 1000 mL) is poured into AC column at a flow velocity of 100 mL/min for 10 min. to adsorb 99mTc on the AC column. A trace amount 99mTc is preferentially and completely adsorbed in the AC column.
- Step(3) Removal of Mo contaminants from AC
- Mo(99Mo) and other nuclides contaminants in AC is removed by flowing H2O, next 6.0 M NaOH (30 mL) and finally H2O.
- Step(4) Elution of 99mTc from the activated carbon
- In order to elute 99mTc collected into AC column, H2O is run through the AC column, then the whole quantity of 99mTc adsorbed on AC column can be eluted.
- Step(5) Removal of Na-ion in alkaline 99mTc eluted
- 99mTc solution obtained in step(4) above is alkaline solution is flowed through to the strong acid type of ion exchange resin (IER) and the activated alumina (AL). By this procedure, Na-ion in eluted 99mTc solution can be taken hold in the IER column and 99mTc can be caught in the AL column. If the IER column is not used, the TcMM process can also be operated by the combination of AC-AL columns system.
- Step(6) Elution of 99mTc
- Finally, a highly pure 99mTc can be recovered from the AL column by flowing 10-20 mL of saline (0.9% NaCl solution), and the resulting 99mTc can be concentrated 50-100 folds from the initial Mo (99Mo) solution.
|
| Producing process of 99mTc | ØEquivalent 99mTc recovery rate in kBq-10TBq |
| ØRecovery of 99mTc : 90~95% | |
| ØConcentration of 99mTc solution: <1Ci/mL | |
| ØProducing time: ≤30 min/run | |
| Quality of 99mTc | Ø99mTc collected in sterile saline |
| ØCollected as 99mTcO4- (pertechnetate) | |
| ØEndotoxin-inspection: negative | |
| ØRadiochemical purity: >4N~7N | |
| ØBn the labeling experiment using many kits, the target medicines are given with high radiochemical purity. |
|
| Waste for production | ØLiquid waste: 250mL/run |
| ØSolid waste: AC 4.5g/run, AL 6~12g/run, | |
| IER 5cc/run, and column casing |
![[Front panel] TcMM10T technetium-1](images/topimg/photo_tcmm.png) |
| [Front panel] TcMM10T |